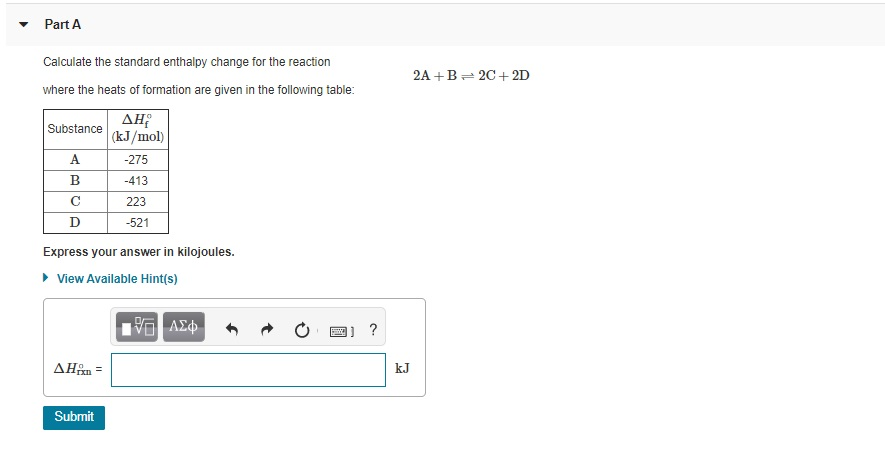
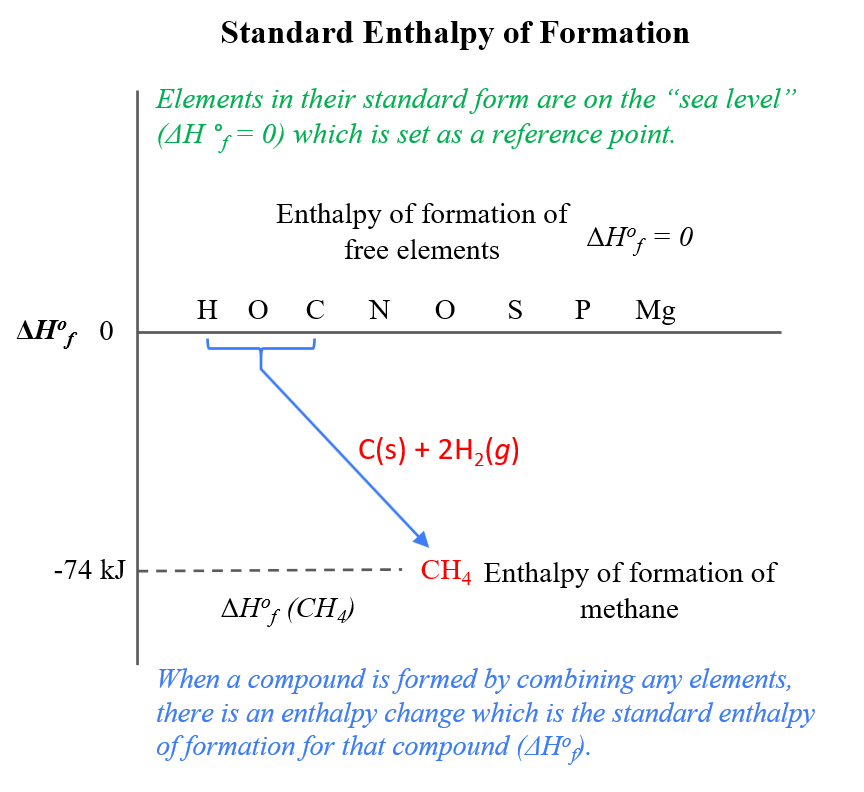
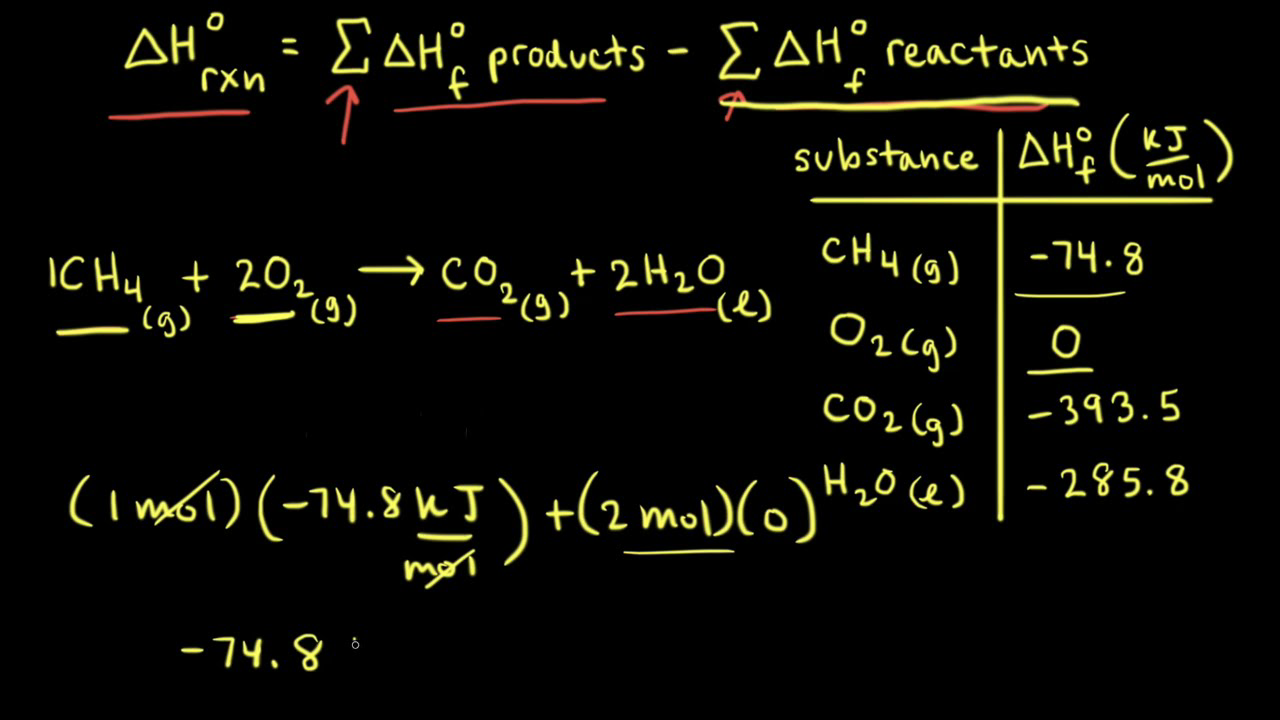
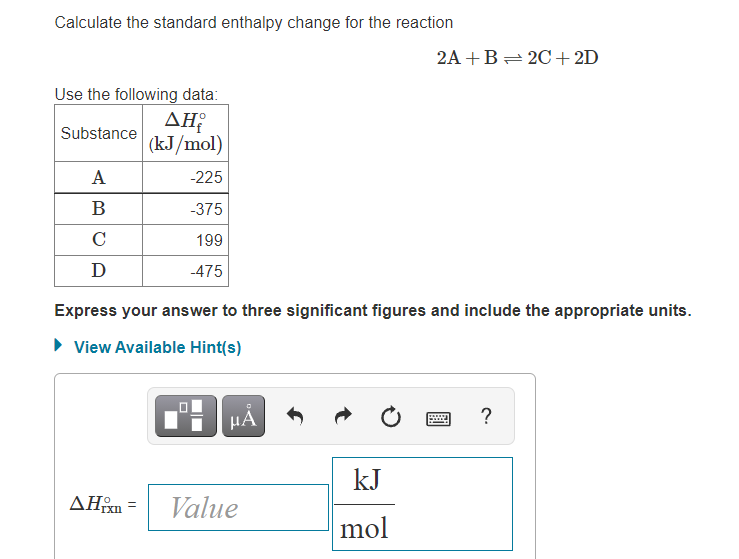
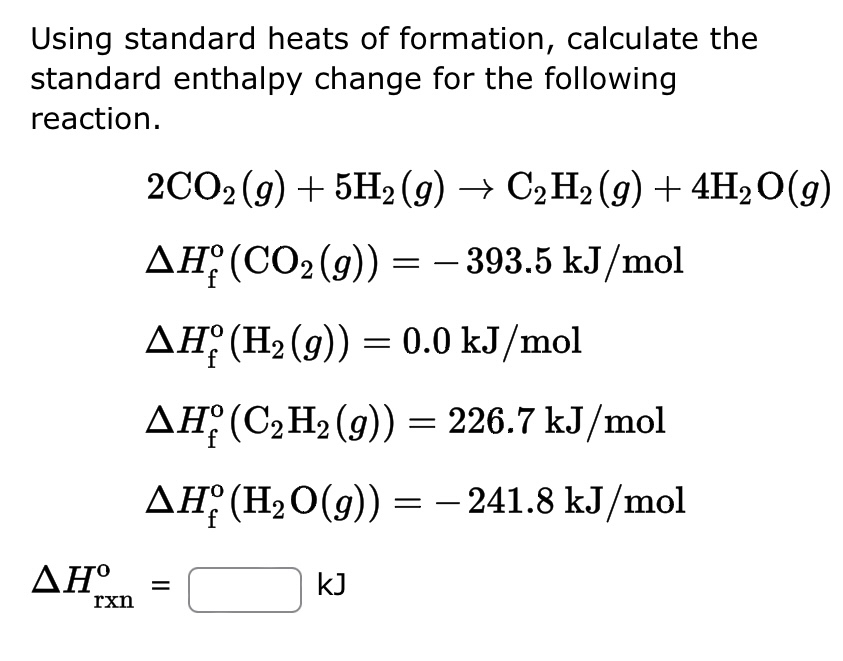
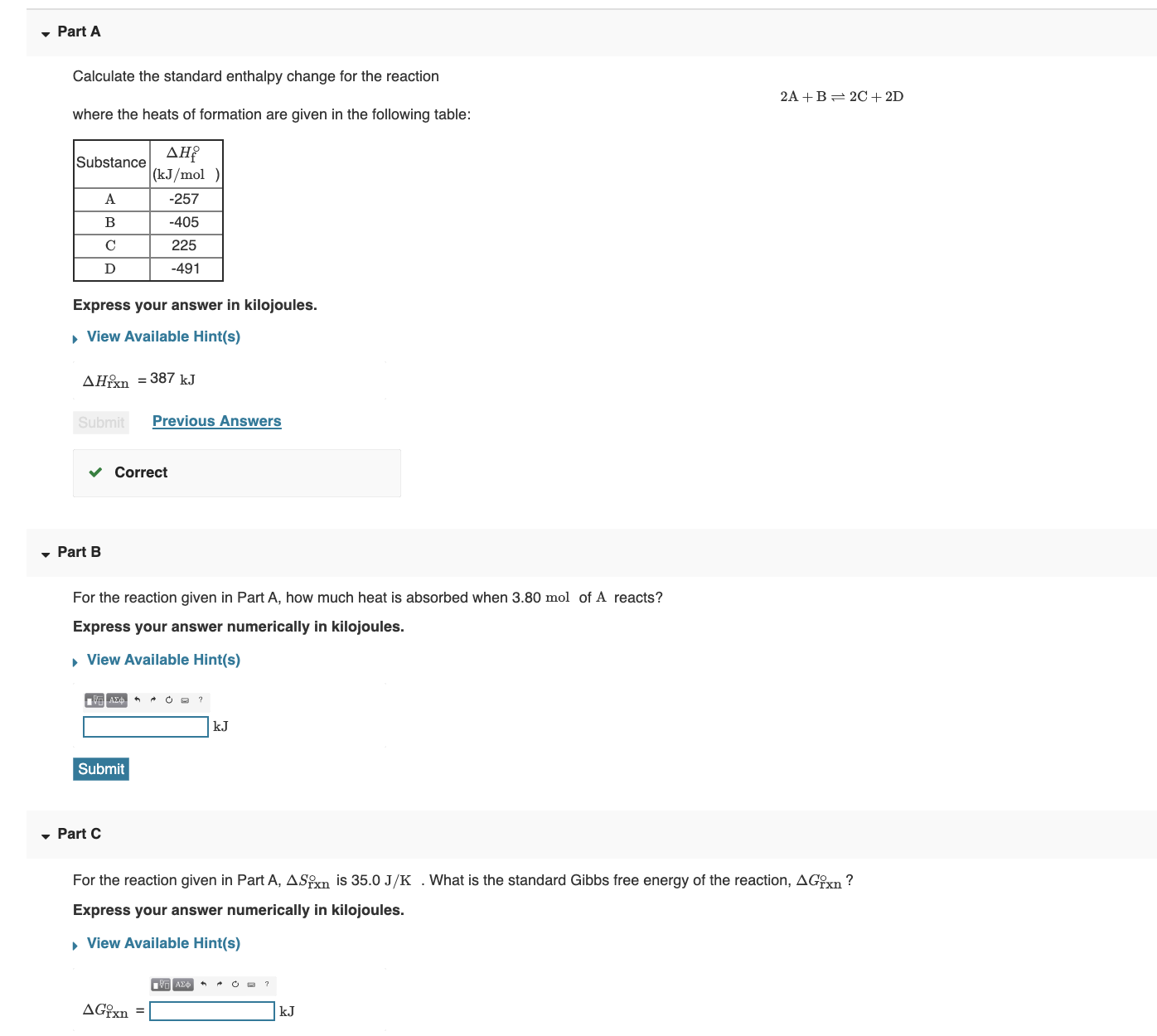
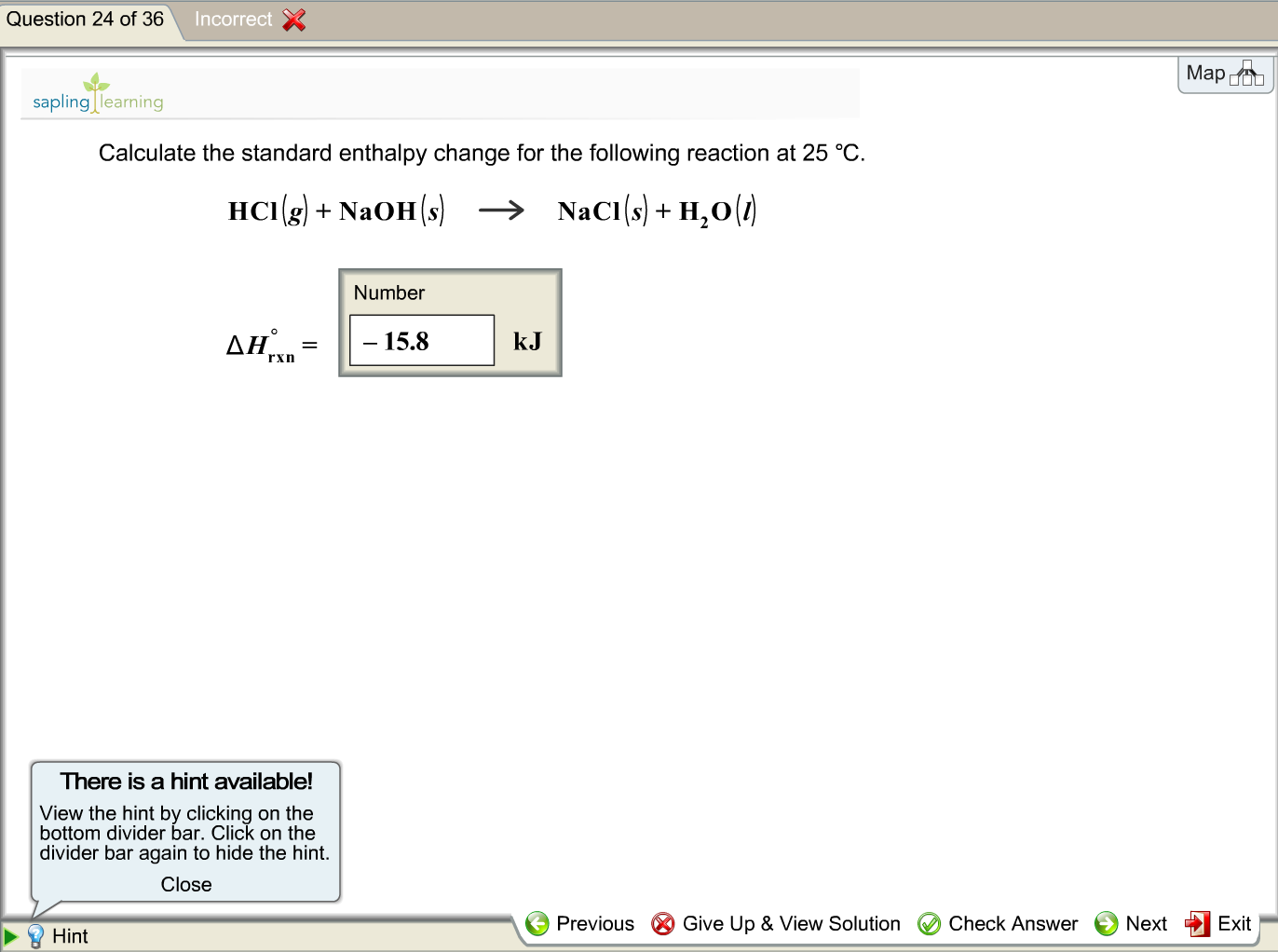
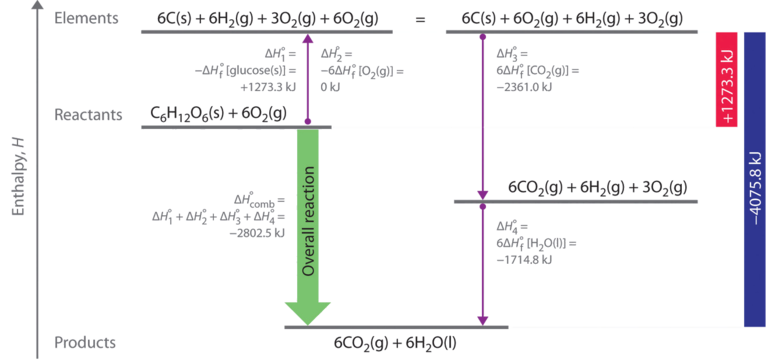
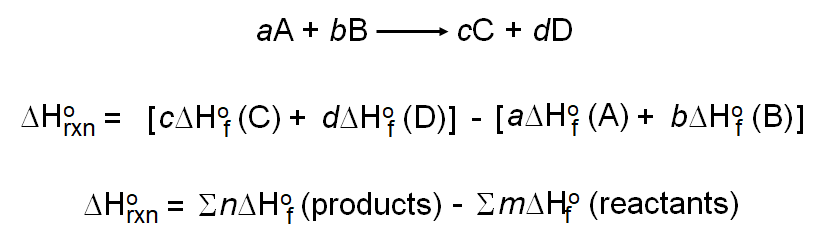OneClass: Calculate the standard enthalpy change for the reaction 2A + B^2C + 2D Use the following d...
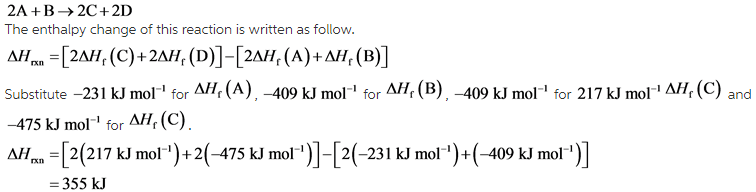
Calculate the standard enthalpy change for the reaction: 2A+B <===> 2C+2D - Home Work Help - Learn CBSE Forum
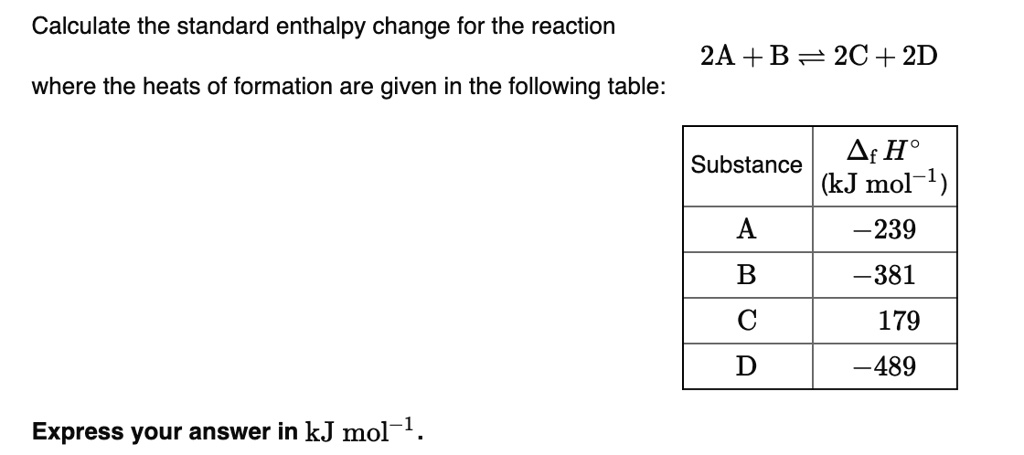
SOLVED: Calculate the standard enthalpy change for the reaction 2A +B = 2C + 2D where the heats of formation are given in the following table: Af Ho Substance (kJ mol-1) A

If the standard internal energy change for the reaction OF2(g) + H2O(g)⟶ O2(g) + 2HF(g) , at 298 K is x kJ . [Given standard enthalpies of formation in kJ mol^-1 are
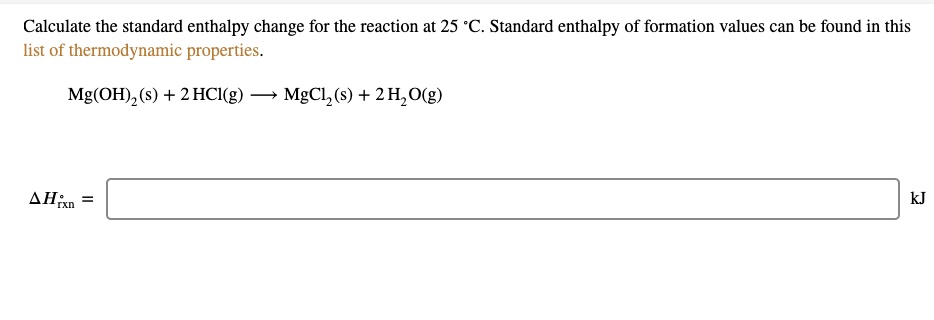
SOLVED: Calculate the standard enthalpy change for the reaction at 25 'C. Standard enthalpy of formation values can be found in this list of thermodynamic properties. Mg(OH) (s) + 2 HCl(g) MgCl,(s) +

Calculate the enthalpy change for the process CCl4(g)→ C(g) + 4Cl(g) and calculate bond enthalpy of C - Cl in CCl4(g) Δ vapH^ (CCl4) = 30.5 kJ mol ^-1 . Δ fH^ (

Question Video: Determining a Standard Enthalpy Change Given the Standard Enthlapy of Fusion | Nagwa